Next Fertility Nordic Clinic (BioEximi OÜ) has recently successfully passed an assessment by the Estonian Accreditation Centre (EAK) for the transition of its renewed medical laboratory to the ISO 15189:2022 standard.
The internationally recognized ISO 15189:2022 standard is crucial for a fertility clinic, as it establishes specific requirements for the quality of laboratory procedures, safety, and staff competence. Compliance with the standard ensures that the clinic operates according to the highest international requirements, maintaining excellence in both reproductive technologies and laboratory practices.
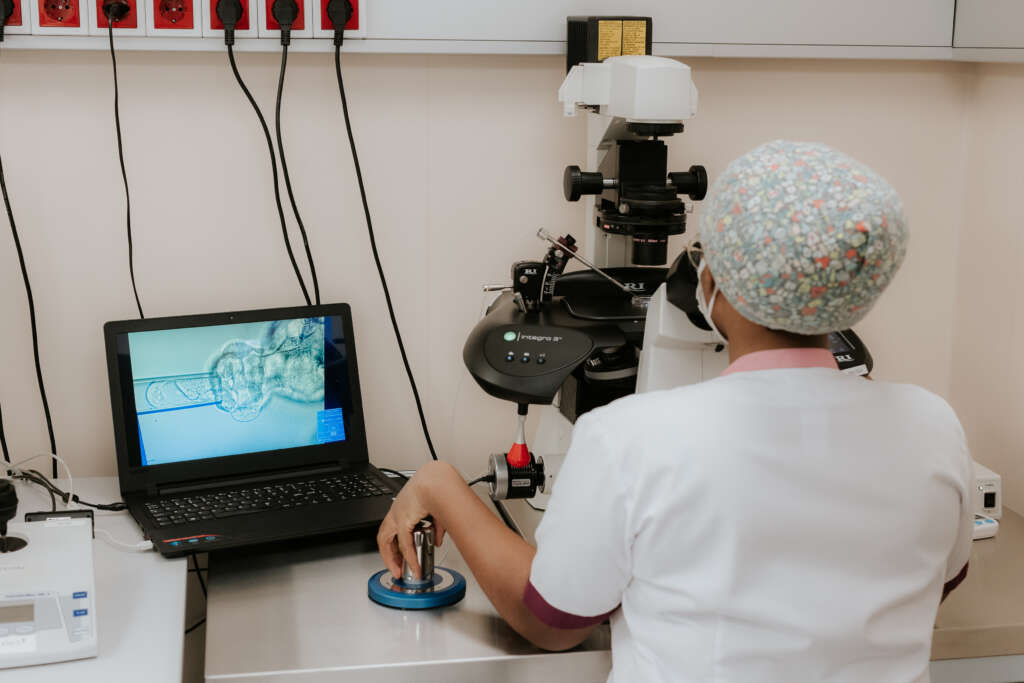
The standard helps ensure that all patient procedures are performed safely and professionally, while also considering ethical and legal aspects. Following ISO 15189 increases patient trust and strengthens the clinic’s reputation as a reliable, quality-oriented partner.
Why is ISO 15189 important?
Patient safety: ISO 15189 standards prioritize patient safety. Adhering to medical laboratory standards ensures that our labs follow strict protocols and procedures to prevent risks and possible errors during procedures, providing a safe environment for patient care.
Quality assurance: Compliance with ISO 15189 standards is a mark of high-quality services. It ensures that every stage of the process, from laboratory practices to patient interactions, meets internationally recognized criteria.
Reliability: It reflects our commitment to excellence, which can increase the confidence and trust of patients seeking assisted reproductive services.
Continuous improvement: ISO 15189 accreditation is not a one-time effort but an ongoing commitment. The clinic regularly evaluates its documentation system and implements recommendations from inspectors to ensure full compliance.
Transparency: We operate transparently regarding our procedures, protocols, and quality control measures. During our annual inspections, evaluators review our standard operating procedures, key performance indicators, and document database.
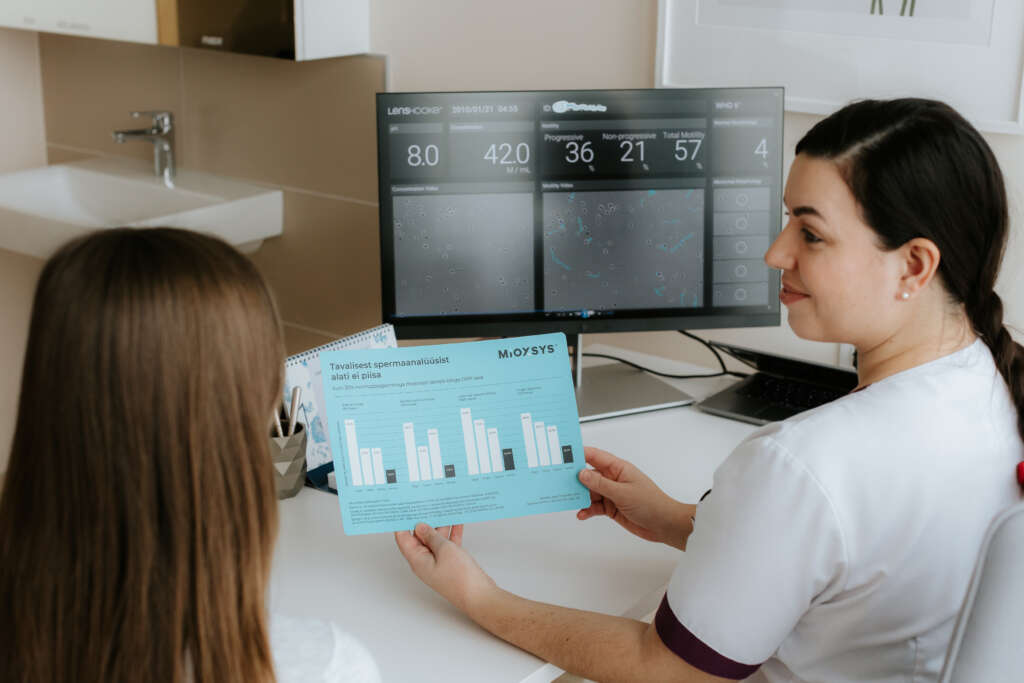
Our ISO 15189 certified methods include:

- Assessment of sperm concentration
- Assessment of sperm motility
- Assessment of sperm morphology
- Assessment of oocyte maturity
- Evaluation of fertilized oocytes or zygotes
- Embryo development assessment (days 2–6)
High treatment outcomes thanks to strict quality control and cutting-edge technology
Next Fertility Nordic is Estonia’s most advanced fertility center, holding the only valid ISO 15189 certificate and using state-of-the-art fertility laboratory technologies:
- EmbryoScope: an incubator where embryos develop undisturbed until day 6
- RI Witness: electronic identification and logging of time and material batches during procedures
- CHLOE EQ: AI-based embryo quality assessment
- Lenshooke X1 Pro: automated sperm quality analyzer
- JRY LoRa sensors: monitoring critical equipment and environmental conditions
- Advanced fertility database (VRepro)
Clean air quality
The IVF laboratory is required to maintain at least D-class air quality. At Next Fertility Nordic, the IVF lab and operating room have achieved C-class air quality. Clean air is ensured by integrated HEPA and LifeAire VOC filters in the ventilation system. Additionally, each incubator is equipped with VOC filters to provide cleaner gas quality inside the incubators.
ISO 15189 is crucial for a fertility clinic because it establishes requirements for procedure quality, safety, and competence. Compliance ensures that the clinic maintains high standards in reproductive technologies, laboratory practices, patient procedures, and ethical considerations. Following ISO 15189 helps guarantee the safety of patients undergoing IVF procedures and preserves the clinic’s reliability among its partners.

