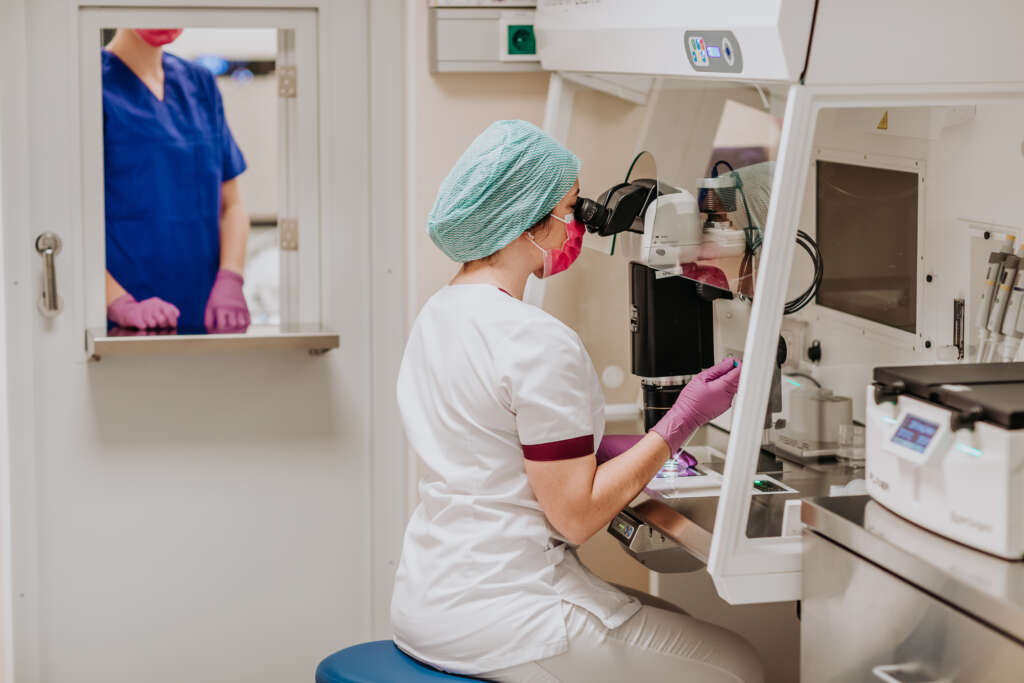
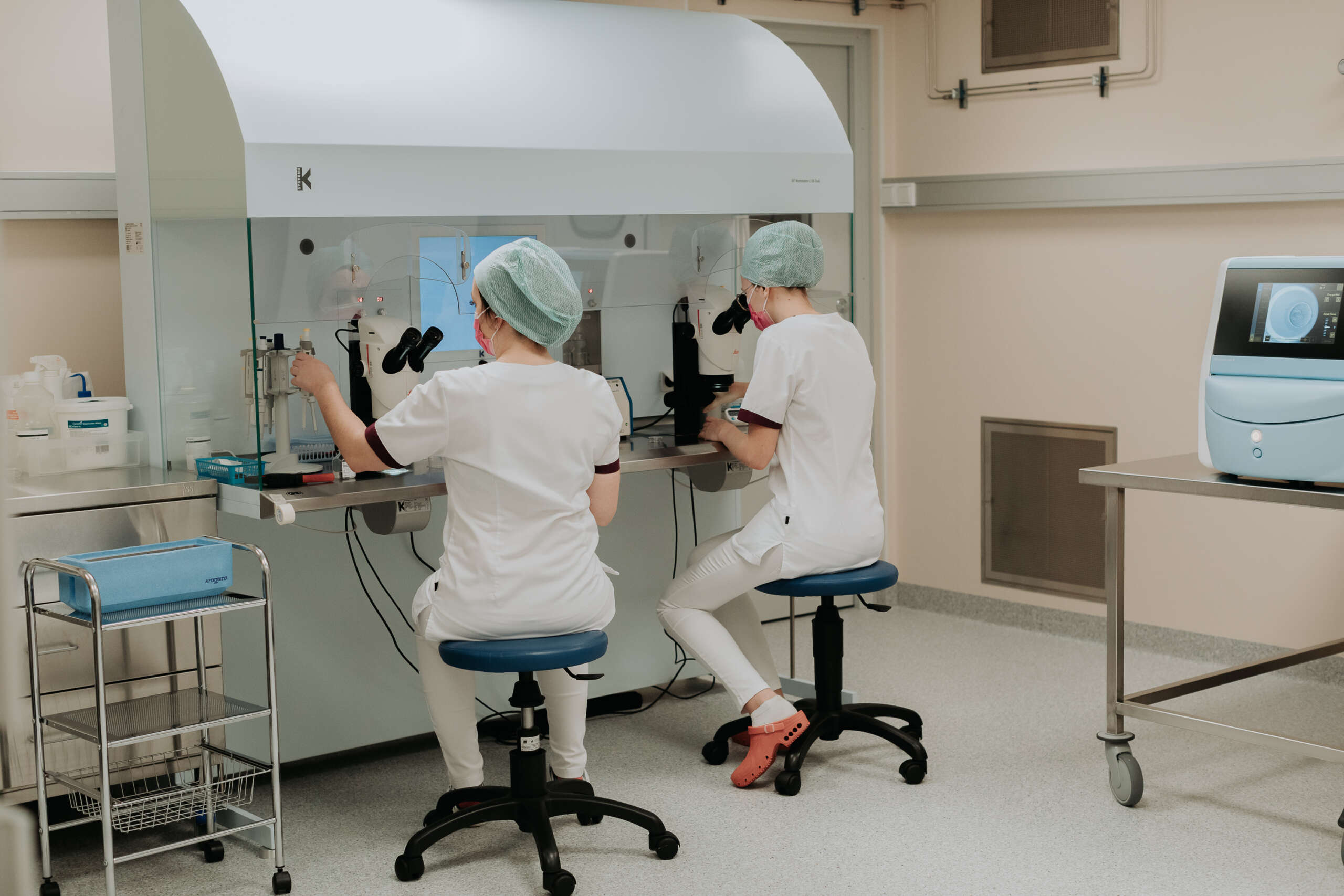
The Estonian Accreditation Centre (EAC) recently recognised the IVF laboratory at the Next Fertility Nordic clinic (BioEximi OÜ) with the ISO15189 quality certificate. This is an internationally recognised quality standard for medical laboratories that evaluates the quality and competence of medical laboratories.
“ In 2021, we moved our clinic and laboratories to new premises in Tallinn, at Veerenni 51,” says Karin Rosenstein, CEO of Next Fertility Nordic Clinic. “When designing the new clinic, we placed great emphasis on the compliance of the laboratory with international quality requirements and equipped our laboratories with the most modern technology to offer the best opportunities in modern infertility treatment. The implemented quality control system describes in detail the management, facilities, equipment and research processes of our organisation. The thoroughly described quality system is sure to help employees in their daily work and ensures continuous traceability of the work process. We are oriented towards improving treatment outcomes and place great emphasis on the continuous training of our employees in order for them to be aware of the latest development trends in the field of infertility treatment.”
“The ISO15189 quality mark further increases trust among our patients and partners. We also prefer to cooperate with companies that follow the quality requirements of ISO15189 in their work, such as Synlab and the Competence Centre on Health Technologies,” adds Rosenstein.
“ We are glad that the Next Fertility Nordic (BioEximi OÜ) clinic applied for accreditation for a medical laboratory according to the EVS-EN ISO 15189:2012,” says EAC Chief Assessor Anastassia Filimonova. “Infertility treatment is a very important field that requires great precision in its work processes and constant monitoring of laboratory conditions. Although applying for accreditation is voluntary in the field of medicine, it certainly helps to increase credibility among clients and is necessary for applying for authorisation from the state to operate in the sphere regulated by legislations.”
In the first year, six methods were accredited in the laboratories of BioEximi OÜ.
-
 Determination of sperm concentration
Determination of sperm concentration
- Determination of sperm motility
- Determination of sperm morphology
- Assessment of oocyte maturity
- Evaluation of fertilised ova or zygotes
- Assessment of embryo development (1-6 days)
Open the certificate