Biopsy technicians Yolanda and Samantha describe the indications for pre-implantation genetic testing (PGT) and the process of embryo biopsy in the IVF lab.


PGT and blastocyst biopsy are cutting-edge techniques that have revolutionized the world of assisted reproductive technology. Prospective parents have the option to screen embryos for genetic abnormalities before implantation, thus increasing their chances of a healthy pregnancy. There are also some practical considerations to understand before proceeding. In this blog, we’ll delve into the world of PGT to describe the process from start to finish.
In a nutshell, PGT involves examination of embryos during an IVF cycle to identify genetic abnormalities before they are transferred to the uterus. There are three types of tests: PGT-A for aneuploidy screening, PGT-SR for structural rearrangements and PGT-M for monogenic disorders. The fertility doctor will discuss which option is right for you in a Gynecologist consultation.
When is PGT recommended?
- Individuals or couples with a hereditary genetic condition
- Both the egg and sperm source are known carriers of the same autosomal recessive disease
- One of the patients has balanced structural chromosomal rearrangements
- A woman is aged 38 or over when she undergoes an egg retrieval
- Recurrent pregnancy loss
- Multiple failed embryo transfers
- Severe male factor infertility
If many good quality embryos are expected to arise, it can help to confirm the order of embryos chosen for transfer and can potentially reduce the number of times an embryo transfer needs to be performed to produce an ongoing pregnancy.
Even if there are no indications, patients can choose to opt in for the service.
How does PGT differ from a ‘typical’ IVF cycle?
- PGT, as an add-on service, should be added to the treatment plan before the start of the cycle day 1. A consultation with a Genetic Counsellor may also be recommended.
- A special consent form will need to be signed prior to taking the biopsy.
- Unlike most ‘typical’ IVF cycles, it is not possible to transfer embryos ‘fresh’ after egg retrieval. Instead, all biopsied embryos are cryopreserved, and the transfer is scheduled in a subsequent frozen-embryo transfer cycle. The Dr may recommend a period (one month) break before the preparation for an embryo transfer cycle commences.
- If there are few follicles or few embryos expected from a cycle, a patient may decide to undergo a second oocyte retrieval to ‘batch’ embryos. If this is the case the lab needs to know ahead of time so the embryos can be frozen on Day 3 of development. During the second cycle, any frozen embryos will be thawed so that the biopsy will occur only once from a combination of both cycles.
What exactly happens to the embryos and cells during biopsy?
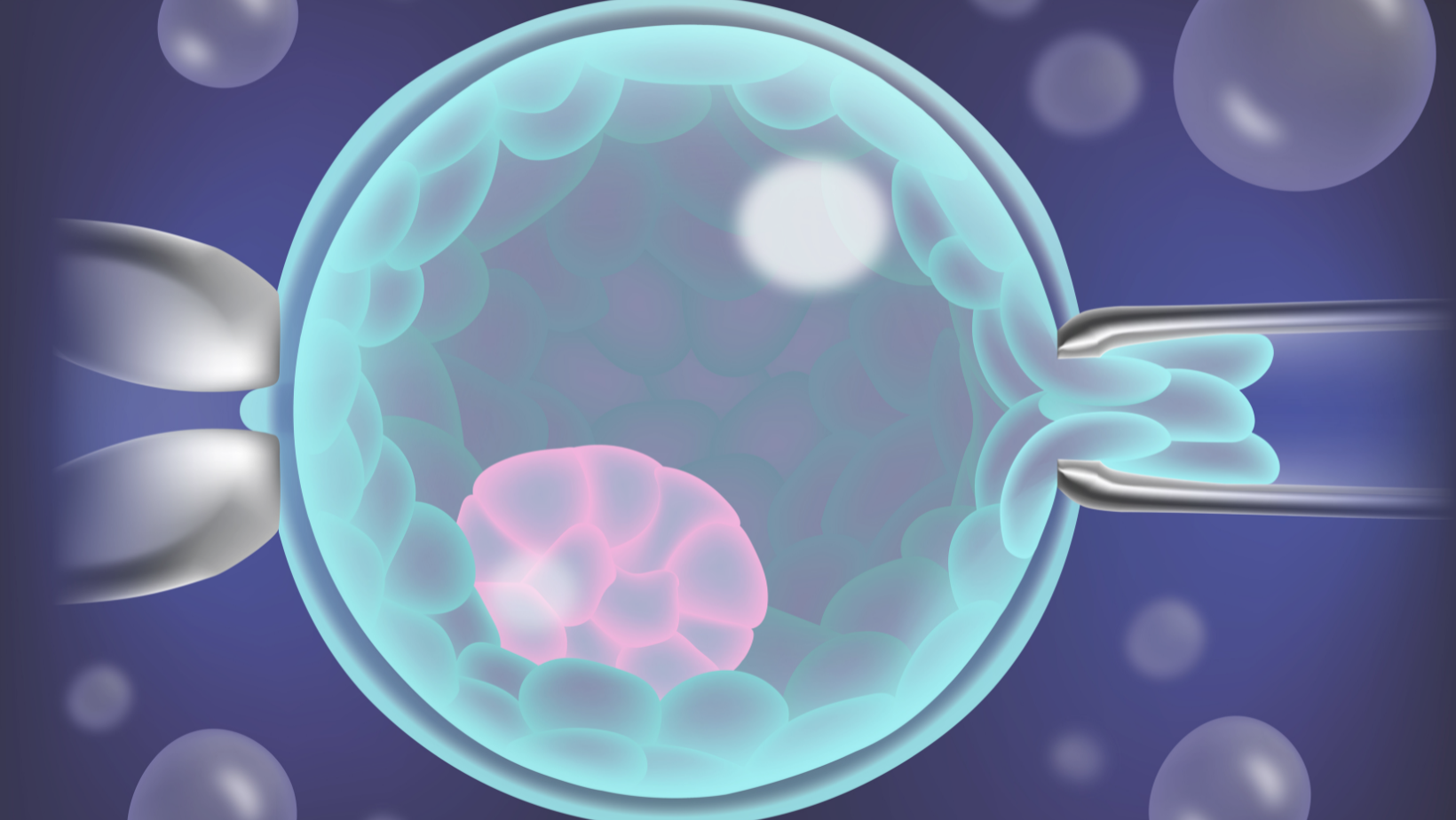
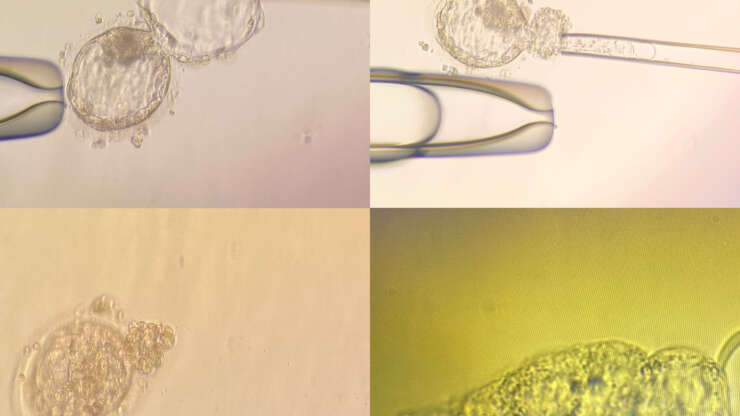
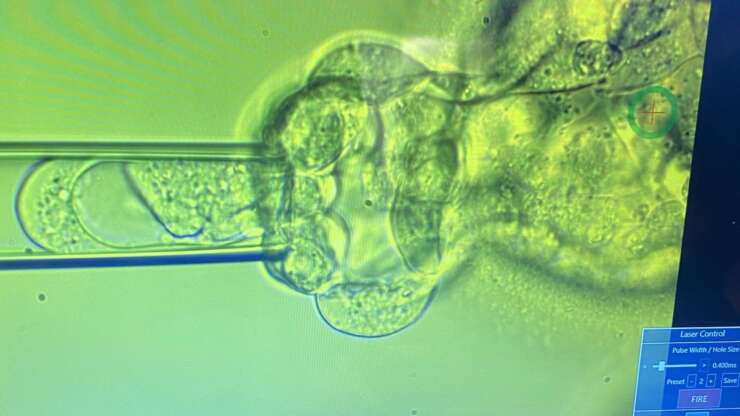
- In our clinic we perform only blastocyst biopsy and on Day 5 and 6 of development. Assisted hatching is sometimes also performed whereby a small opening in the zona is made using a laser. This allows for cells to protrude away from the embryo.
- Only embryos that Embryologists consider ‘suitable for biopsy’ are used. CC (poor quality) embryos are not recommended for testing.
- 4-8 cells from the outer edge of the embryo are aspirated using a fine pipette and they are pulled away from the rest of the embryo.
- The aspirated cells are placed into a small tube under a microscope and kept at -20 degrees.
- The remaining embryo(s) are cryopreserved in the normal way according to biopsy number.
- The extracted cells are sent to a genetics lab. These cells represent the genetic make-up of the remaining cells of the embryo.
- The results can take up to 2 to 3 weeks to be sent to the medical professionals.
- Once the PGT results are available, the Doctor will inform the patients with a recommendation on how to proceed.
How much does age impact the likelihood of normal embryos?
The average rate of having an aneuploid embryo while a woman is under the age of 35 is 30%. At age 44 or older, this increases up to 90%!
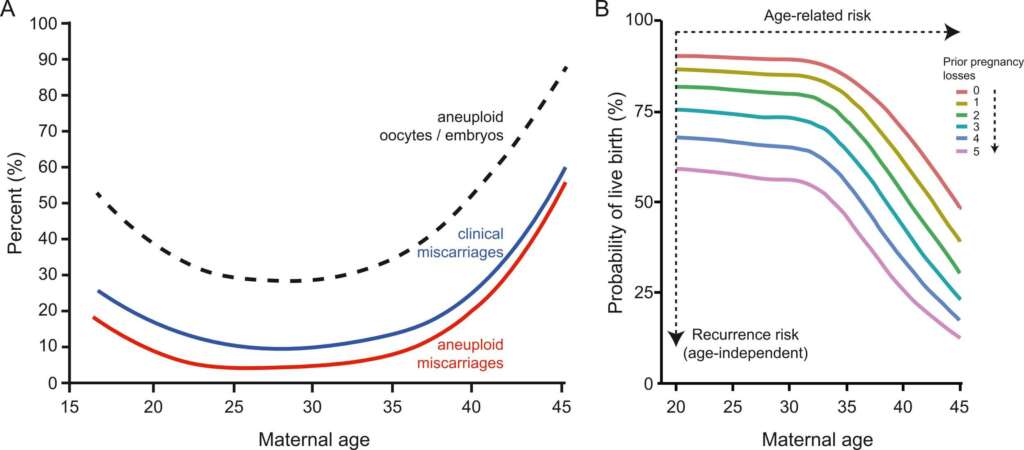
According to general statistical data, the live birth rate of a euploid embryo is around 50% independent of a woman’s age.
https://doi.org/10.1016/j.semcdb.2022.01.007
What’s the difference between PGTA/ PGTSR/ and PGTM?
PGT-A (Pre-Implantation Genetic Testing for Aneuploidy):
PGT-A is the most common form of testing. It detects numerical chromosomal abnormalities. There may be an addition or deletion of a whole chromosome (including the sex chromosomes X and Y). No preparation from the patient side is needed.
PGT-SR (Pre-Implantation Genetic Testing for Structural Rearrangements):
Before planning a PGT-SR cycle, the patient must perform a karyotype. If a karyotype is normal, the test cannot be undertaken.
PGT-M (Pre-Implantation Genetic Testing for Monogenic Disorders):
Before planning a PGT-M cycle, clear documentation of the known monogenic genetic disease in the patient(s) and any family tree evidence is needed. A gene panel and a genetic report will be ordered and the results saved. The genetic laboratory will review the records and ensure that they can proceed with the test. 6 weeks in advance, blood sample kits need to be sent to the genetics lab for proper test preparation. If possible, a saliva sample from any living parents should also be arranged. If this is not possible, our lab may be required to send discarded reproductive material along with the cells so that enough DNA is collected for an accurate test. Some examples of monogenic diseases: cystic fibrosis, sickle cell anemia, or Huntington’s disease.
PGT-A will also be performed on any unaffected embryos. That means you can have an unaffected embryo for the specific gene analysed, but it can still be chromosomally abnormal.
What to expect when the results come back?
There are different possible categories:
Euploid (PGT-A):
- Embryos are normal, this is a positive outcome. The patient can proceed with the transfer of these embryos, which typically increases the chances of a successful pregnancy.
Aneuploid (PGT-A):
- Embryos are abnormal. These embryos are not suitable for transfer, as they have a higher risk of implantation failure or miscarriage. The patient may need to consider additional IVF cycles or other options, such as using donor eggs/sperm.
Aneuploid Chaotic (PGT-A):
- Results show six or more chromosome changes identified. The predictive value of a chaotic aneuploid result may be reduced. Re-biopsying the embryo to confirm the result can be considered at the clinic’s discretion.
Indeterminate (PGT-A):
The genetic laboratory was not able to obtain a reliable result for the embryo. This can happen if there is not enough DNA in the biopsy sample or the quality of DNA is not sufficient to get a result. The embryo could be chromosomally normal or abnormal and is now considered as an ‘untested’ embryo. Re-biopsy can be considered at the clinic’s discretion and according to embryo quality.
Mosaic (PGT-A):
- There is a mix of euploid and aneuploid cells in the biopsy. In the absence of euploid embryos, it is possible to transfer ‘low-mosaic’ embryos after genetic consultation.
Balanced (PGT-SR):
- For PGT-SR, the results might reveal embryos with balanced chromosomal rearrangements. These embryos carry the same genetic information as the parents but in a balanced form. These embryos can be considered for transfer, as they are not at an increased risk of genetic abnormalities.
Unbalanced (PGT-SR):
- PGT-SR can identify embryos with unbalanced structural chromosomal rearrangements. Unbalanced embryos are generally not recommended for transfer, as they have an increased risk of birth defects and developmental issues.
Low risk (PGT-M):
- The embryos do not carry the specific genetic mutation for the targeted monogenic disorder. These embryos are suitable for transfer and have a reduced risk of inherited disorder.
At risk (PGT-M):
- The potential child is at risk of inheriting the targeted monogenic disorder. In some rare cases, these embryos may be considered if no alternative unaffected embryos are available, depending on the severity of the genetic condition.
Are there any potential limitations of PGT?
- While genetic testing is highly accurate (98%), it is not infallible. There is a small possibility (2%) of false- positive or false-negative results, which can lead to incorrect conclusions about a genetic condition or embryo quality.
- Adding PGT testing, along with the IVF process itself comes at an extra cost.
- There is a chance that patients may not have any embryos suitable for transfer and this can come as an emotional burden.
- Although the process of embryo biopsy is generally safe, it is an invasive procedure so there is no guarantee that the embryo(s) will not have been harmed or that they will survive the freeze and thaw process before embryo transfer, especially in instances of re-biopsy (where the embryo will have been frozen 2 times). The risk is still very minimal.
- The gender of the embryos are not revealed to patients. Sex selection is prohibited in Estonia. Only when there is a sex-linked disorder in question (PGT-M) will the gender be selected for transfer.
A patient-friendly visual demonstration on the process is shown in this video:
Day 5 embryo biopsy